MANTROCEL® HPMC is a semi-synthetic, water-soluble derivative of the naturally occurring polymer cellulose. It is widely used as a coating agent, thickener, binder, emulsifier and suspending agent in pharmaceutical and cosmetic formulations, as well as in food products. It excels because of its ease of handling, good safety profile and the availability of a broad variety of different grades that cover a wide range of applications.
MANTROCEL® can either be used in an aqueous solution or in its powdered form. After swelling, it dissolves freely in water and forms gels of different viscosities, depending on the concentration and grade of MANTROCEL® used.
As a solution, MANTROCEL® can be used as an excellent gel former and thickening agent for topical, nasal and ophthalmic formulations for pharmaceutical, cosmetic and food products. Furthermore, it is a valuable inert coating agent for masking the odour and taste of drug substances in tablets, to protect drugs and nutritional supplements from deterioration and to give tablets and capsules an aesthetic film coating. A solution of the product can also be used as a base to formulate colour coatings.
In its powdered form, MANTROCEL® is used in low concentrations as a binder for tableting and dry-granulation, or as matrix former for sustained-release tablets.
The different grades of MANTROCEL®
| MANTROCEL® |
|
|||||||||||||||||||||
|
|
Synonyms: Hypromellose; Hydroxypropyl Methyl Cellulose; Cellulose, 2-hydroxypropyl methyl ether
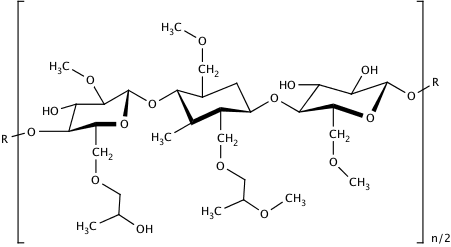
HPMC is a white/off-white odour- and tasteless powder. Chemically, it is a partly O-methylated and O-(2-hydroxypropylated) cellulose and is available in several grades that differ in molecular weight, and therefore also viscosity, as well as the extent of substitution.
|
| HPMC, Hypromellose |
MANTROCEL® is available in two types of substitutions: E and K. The E type corresponds to Hypromellose 2910 and the K type to Hypromellose 2208, according to the EP and USP. The first two digits refer to the percentage (w/w) of the methoxy, while the second two digits refer to the percentage of the hydroxypropoxy-groups in the dried substance. Due to their lesser substitution, the K types can be considered more cellulose-like and slightly less swellable compared to HPMC E. In general, the K-types are more often used for sustained release applications, while E-types are more frequently employed for film coating. Both types are employed for viscosity regulation and gel formation. In addition to the substitution pattern, the different HPMC grades can be distinguished by the apparent viscosity (mPas) of a 2% (w/w) aqueous solution. The viscosity range reaches from 3 mPas up to 100 Pas, which corresponds approximately to a molecular weight of 10 kDa to 1500 kDa. The lower viscosity grades (up to 15 mPas) are preferred for film coating.
MANTROCEL® is produced from renewable, vegetable sources by chemical modification. Purified cellulose is activated by the addition of NaOH and then further treated with chloromethane and propylene oxide to obtain Hydroxypropyl Methylcellulose. The obtained HPMC is directly purified, dried and milled, or is treated in advance with HCl to reduce the viscosity of the HPMC by depolymerization.
Applications of HPMC
Depending on the grade, MANTROCEL® can be used in many different applications:
- binder for direct compression
- binder for granulation
- gel-forming agent in hydrogels
- coating for immediate release and taste and smell masking
- matrix in sustained release tablets
- amorphous solid dispersions (AMD)
- stabiliser in macro- and nano-suspensions
MANTROCEL® powder shows sufficient flow properties and is easily miscible with most other tableting excipients and a range of API's. Since it absorbs moisture after opening of the original packaging, it should preferably be used within a short time and under controlled humidity. When HPMC is used in an aqueous dispersion, no precautions have to be taken, but weight change of the material due to water uptake should be considered.
Aqueous HPMC Solutions
All grades of MANTROCEL® are easily soluble in cold and ambient-temperature water, but its solubility decreases in hot water. At a certain temperature, the so-called cloud point, HPMC undergoes a sol-gel transformation that is fully reversible and depends on the type of HPMC, its concentration and the ion type and strength in the aqueous medium. For a 2% MANTROCEL® K15 solution in purified water, the cloud point is around 71 °C; however, the addition of Na2SO4 (0.8 mol/L) reduces it to less than 30 °C. This effect can be used for the easy preparation of solutions, but may also lead to the altered release profiles of HPMC coated drug forms or from matrix tablets after oral application, depending on food intake.
MANTROCEL® solutions can be made by simply dispersing the powder in cold water. After dispersion, the powder particles need some time to swell and form a clear solution or gel. Several methods are valuable to avoid lumping of the particles, which will slow down the forming of the solution. Foam formation can be reduced by avoiding high shear forces or by using vacuum mixing units. If necessary, anti-foam agents can be added at a minimum concentration to avoid any interference with the performance of MANTROCEL®.
1. The MANTROCEL® powder can be dispersed in cold or ambient-temperature water by employing a high-shear mixer for disintegrating lumps.
2. MANTROCEL® is dispersed in hot water (approx. ¼ of the final desired volume) close to 100 °C. At this temperature it is less soluble and the single particles can be more easily separated. After thorough agitation, the rest of the cold water is added to reduce the temperature and induce swelling of the polymer.
3. 3. Since the solubility of HPMC is also dependent on the salt concentration, an initial dispersion of HPMC in a concentrated salt solution can be formed to facilitate dispersion. The solution is obtained after dilution with cold water to the desired ion strength.
4. For smaller quantities, it can be suitable to disperse MANTROCEL® on the surface of the cold aqueous medium and allow it to swell for several hours in the fridge. Then, the swollen HPMC is fully dispersed in the medium to obtain the final solution.
Safety of HPMC
HPMC is widely used as an excipient in pharmaceutical, cosmetic and food products and is GRAS listed by the FDA and approved as a food additive in the EU (E 464). Since the average consumption of HPMC is very little, no acceptable daily intake has been defined by the WHO. It is only known that excessive consumption might have a laxative effect.
In an animal study with ultra-low viscosity HPMC, it was shown that following oral ingestion, the fraction reaching the blood circulation was negligible. In addition, HPMC is barely modified by intestinal microorganisms. Any adverse effects of orally consumed HPMC are therefore limited to its physical effects in the gastro-intestinal tract. Due to its ability to retain water and its indigestibility, it can cause a laxative effect in concentrations of around 5 g/person/day. HPMC may influence the absorption of other chemical or nutritional substances from the intestine, mostly by increasing luminal viscosity. In a two year long-term study, rats were fed up to 20% HPMC in their daily diet and no adverse reactions were observed. The LD50 in mice and rats after intraperitoneal administration was determined to be around 5 g/kg. Studies regarding sensitization and irritation following dermal and ophthalmic application are only available for hydrophobically modified HPMC. This type of HPMC was found to be not-sensitising and only a mild eye irritant. Considering this and the long use of HPMC in ophthalmic and topical formulations, any possible sensitization or irritation can be seen as negligible.
In conclusion, MANTROCEL® can be considered as safe for use in all types of pharmaceutical, cosmetic or food products.
| Packing | E-Grades: 20 kg box lined with polyethylene bag |
| K-Grades: 25 kg drum lined with polyethylene bag | |
| Shelf life | E-Grades: 5 years |
| K-Grades: 3 years | |
| Storage | At ambient temperature, protected from moisture. |
| Specifications MANTROCEL® E5 | |
| Specifications MANTROCEL® E6 | |
| Specifications MANTROCEL® E15 | |
| Specifications MANTROCEL® E50 | |
| Specifications MANTROCEL® K4M | |
| Specifications MANTROCEL® K100M | |
| MSDS |


