Vitamin E TPGS, Tocofersolan (INN), is a water-soluble derivative of natural Vitamin E. It is a surfactant containing both lipophilic and hydrophilic groups in the molecule. Originally developed for the treatment of Vitamin E deficiency in patients with malabsorption, it is now used as an effective enhancer in formulations to increase bioavailability of lipophilic and poorly soluble substances. Vitamin E TPGS has unique properties and a long-established safety profile in pharmaceuticals, cosmetics, foods and animal feed.
Information on how to dissolve vitamin E TPGS for oral administration can be found here.
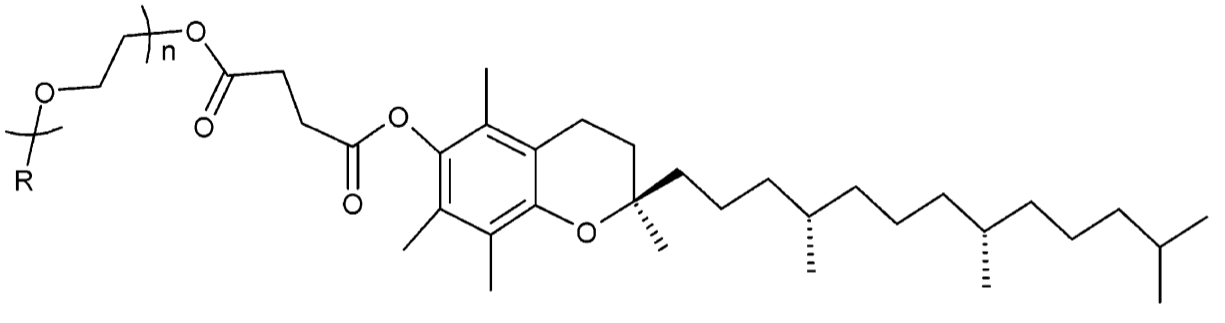
Molecular structure of Vitamin E TPGS
Pharmaceutical applications

Vitamin E TPGS has a documented benefit in the solubilisation and significant improvement in absorption, bioavailability and effectiveness of drugs.
Main applications in the pharmaceutical industry include:
- Solubilisation and formulation of lipophilic and poorly soluble APIs including cyclosporine, amprenavir and paclitaxel
- Drug delivery and control release of APIs
- Increased absorption and bioavailability of poorly soluble APIs
- Oil in water emulsifier
- Vitamin E supplementation in malabsorbing patients
Cosmetic applications

Vitamin E TPGS is used in personal care and cosmetic products as water-soluble Vitamin E and as an enhancer in the formulation of other actives. It serves as ethanol-free, hypoallergenic, non-irritating emulsifier/ excipient in personal care and cosmetic products and can be also used for the solubilisation of poorly soluble drugs. The INCI name of Vitamin E TPGS is Tocophersolan.
The main applications in cosmetics and personal care products include:
- Eye drops, nasal sprays, syrups and others primarily in formulating active compounds.
- Creams, lotions and other products for topical application of Vitamin E and other formulated actives.
Use in Nutritional Supplements
In the US, Vitamin E TPGS can be used as a source of Vitamin E in all dietary supplements and foods. In Europe, Vitamin E TPGS is explicitly approved for use in "food for special medical purposes" in the Annex to Article 15 of Regulation (EU) No 609/2013.
Unlike natural Vitamin E, Vitamin E TPGSS does not have to be emulsified by bile salts in the intestine to become absorbed. It can therefore also be used as a source of Vitamin E in groups with poor fat digestion, e.g., after gallbladder removal or in the case of cystic fibrosis (mucoviscidosis). In addition, it increases the bioavailability of other fat-soluble vitamins (A, D and K) as it forms water-soluble micelles with these, which are well absorbed by the body. Vitamin E TPGS is derived from the natural form d-alpha-tocopherol and therefore has a relatively high content of 372-447 IU/g d-alpha-tocopherol. In addition, a combination of this product with natural Vitamin E can be useful, as Vitamin E TPGS also increases its bioavailability in the same way as the other fat-soluble vitamins.
| Specifications | |
| MSDS |


