EASTMAN™ CA
Cellulose Acetate, Sustained-Release
EASTMAN™ CA is commonly used in osmotic (controlled-release) drug delivery and taste-masking. It can be used as film coating, as matrix former in direct compression of tablets or as polymer matrix in solid dispersions. EASTMAN™ CA is made under cGMP and meets the requirements of the National Formulary (NF) and European Pharmacopoeia (EP). It is the subject of U.S. Drug Master File 9323.
Two pharmaceutical grades are available:
|
| Form |
Viscosity
(P) |
Acetyl-
(%) |
DoS1) |
Hydroxyl-(%) |
TM(°C) |
TG(°C) |
MWn2) |
|
|
|
| Pellets |
2.1 |
32.0 |
1.8 |
8.7 |
230-250 |
213 |
36,000 |
| Powder |
38.0 |
39.8 |
2.4 |
3.5 |
230-250 |
191 |
18.500 |
|
1) Degree of Substitution
2) Number-average absolute molecular weight in THF (for CA-398-10) and NMP (for CA-320S)
Sustained Release Through Permeable Membranes
The osmotic pump drug delivery technology typifies sustained release that capitalizes on the nature of
EASTMAN™ CA films — insoluble yet semipermeable — to allow water to pass through a tablet coating. An osmotic agent that swells absorbs the water, forcing the active out through a hole drilled in the film.
The same effect can also be achieved by employing water-soluble materials, e.g. hydrophilic plasticizers like PEG 400 or TEC, in the film to increase the drug’s ability to diffuse through it. The degree of acetylation of cellulose acetate
has a significant effect on the water permeability of its film. The higher acetyl content in the cellulose acetate
will give lower permeability. Therefore, the drug release rate can be adjusted by mixing the two types of
EASTMAN™ CA, where
CA-320S leads to a faster and
CA-398-10NF to a slower release rate. Additionally, the
glass transition temperature (T
g) decreases and the stretchability increases as the degree of substitution of
cellulose acetate esters increase.
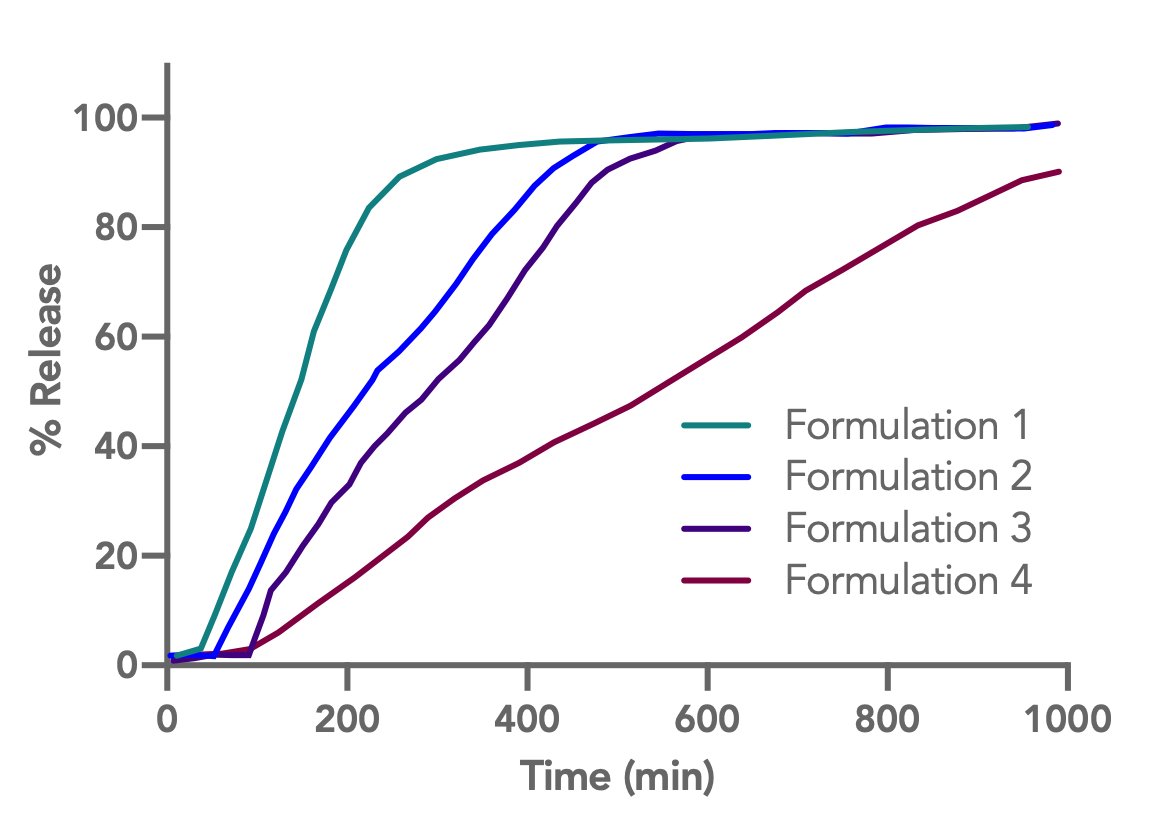
|
| Ibuprofen release profiles from CA-398-10NF/EP coated bi-layer osmotic tablets
|
Coating formulations for bi-layer osmotic tablets
Sustained Release by Direct Compression
Sustained release from direct-compression matrices of
CA-398-10NF has been demonstrated for both
relatively water-insoluble and very water-soluble actives. The methodology employed consisted of mixing
plasticizer, if any, with
CA-398-10NF followed by incorporation of the active with additional mixing. On one hand, the release rate can be adjusted by the ratio of active to polymer, on the other hand by the addition of
plasticizers or
CA-320S. This way, basically any drug release profile can be realised.
Solid Dispersions
A relatively new application for
EASTMAN™ CA is the use in solid dispersions to obtain a sustained release profile for poorly soluble drugs.
EASTMAN™ CA increases the solubility of the active and decreases the drug release rate at the same time. The desired release profile can be adjusted by the addition of hydrophilic excipients or other polymers.
Packing and storage
| Packing: | 49.8 kg (CA-398-10NF)/ 45.3 kg (CA-320S) fiber drum with inner PE-lining |
| Shelf life: | 4 years |
| Storage: | At ambient temperature, protected from moisture. |
Identifier
| CAS-No.: | 9004-35-71 |
| INCI: | Cellulose Acetate |
Material Safety Data Sheet for EASTMAN™ CA
Further product information can also be found via the following links:
You are also welcome to contact us directly at any time.





