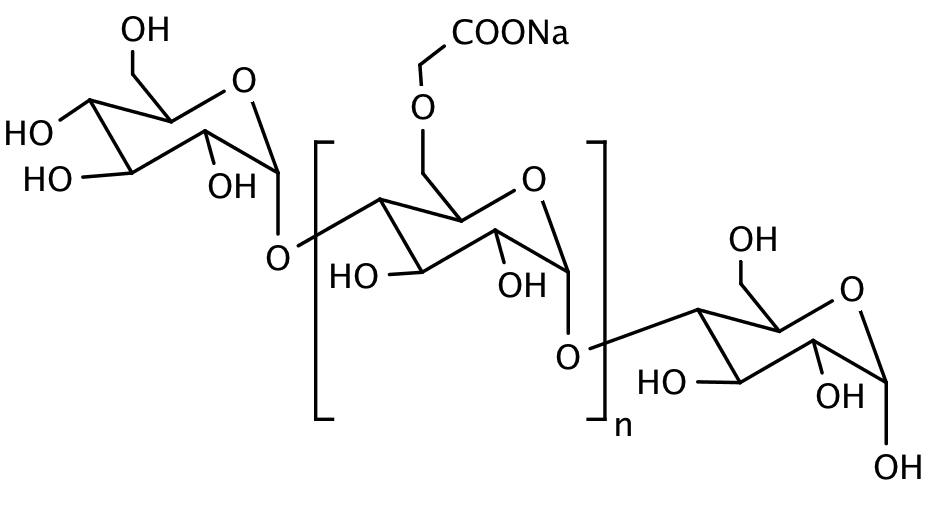
ULTRAMYL is an excellent tablet disintegrant. Chemically, it is a low substituted Sodium Carboxymethyl Starch, also known as Sodium Starch Glycolate, with the following structural formula:
The combine interaction and mechanism of ULTRAMYL ensures a rapid and complete disintegration of the tablet as soon as it comes into contact with water. A significant decrease in the disintegration time can be achieved in almost all formulations by addition of ULTRAMYL. The only exceptions are formulations with highly soluble substances.
Extensive experience in the research laboratory have demonstrated excellent disintegrating effect of ULTRAMYL in tablet formulated by either dry or wet granulation or by direct compression. In the conventional granulation technique, ULTRAMYL is mixed extra granularly.
The exceptional disintegrating effect of ULTRAMYL is due to the following properties
- - immediate water intake
- up to 300% increase in particle volume
- large molecular expansion
- complete cleavage of intermolecular bonds
One of the most significant properties of ULTRAMYL is the retaining of its particle shape upon swelling. Since the particle doesn’t form any soluble components, there is little tendency for gelatinisation that is known to occur with other disintegrants with extended disintegration time.
ULTRAMYL offers the following key benefits:
- - good compressibility, in almost all formulations
- uniform particle size distribution
- high density
- low percentage in total formulation
- high stability
ULTRAMYL has excellent long term storage stability with good disintegration activity. The optimum concentration of ULTRAMYL in a formulation is in the range of 2% - 4%, calculated based on tablet weight. Depending on the formulation, a concentration less than 1% can be sufficient. It must be noted that it is not recommended to exceed more than 8% as this usually leads to a deterioration of the disintegration time. The optimum concentration has to be identified for each formulation by appropriate tests.
Even though ULTRAMYL has the same particle size distribution as potato starch, it still exhibits excellent flow properties and improves powder flowability of the entire formulation.
The high bulk density of ULTRAMYL ensures that the filling density of the tablet formulations does not increase even if ULTRAMYL is used in the same weight as any other disintegrant. In contrast to starch, microcrystalline cellulose or alginic acid, ULTRAMYL does not affect the flow of powder blend and it has good compression properties. These two features make ULTRAMYL an ideal disintegrant for direct compression.
When used with moisture-sensitive API, ULTRAMYL is always preferable compared to untreated starch. At 50% relative humidity, ULTRAMYL equilibrates to a moisture content of about 10%, while it is usually over 20% for starch. Since the quantity required for ULTRAMYL is much lesser, the moisture content of the total formulation can be significantly reduced.
Sodium Carboxymethyl Starch is included in the European Pharmacopoeia (Ph. Eur.) and as Sodium Starch Glycolate in the National Formulary (NF). ULTRAMYL complies with the specifications and regulations from the EU or US Pharmacopeia.
| Packing | 25 kg PE bag in paper box |
| Shelf life | 4 years |
| Storage | At ambient temperature, protected from moisture. |
| Electron Micrograph | |
| Specifications | |
| MSDS |


